Abstract
Context. A 17-gene Leukemic Stem Cell gene expression signature (LSC17) has been shown to be prognostic in AML (Ng et al., 2016), independently of genetic risk stratification (Bill et al., 2020; Duployez et al., 2019). Whether assessment of LSC17 adds prognostic value on top of measurable residual disease (MRD) has not been explored.
Objective. We analyzed the prognostic value of LSC17 in adult AML patients prospectively treated in the intensive chemotherapy ALFA-0702 trial (NCT00932412) in the context of ELN 2017 risk stratification (ELN17) and NPM1 MRD.
Methods. The LSC17 score was determined by Nanostring technology (Duployez et al., 2019). NPM1 MRD negativity was defined as a 4-log reduction in peripheral blood NPM1c expression (Balsat et al., 2017; Lesieur et al., 2021). Multiple testing was adjusted for using the Benjamini-hochberg procedure.
Results. The study population included 504 AML patients (237 female, 267 male) with a median age of 48 years (range 18-60). One hundred eighty-seven (37%) patients had an NPM1 mutation. ELN17 risk was favorable in 176 (35%), intermediate in 151 (30%) and adverse in 170 (34%) patients.
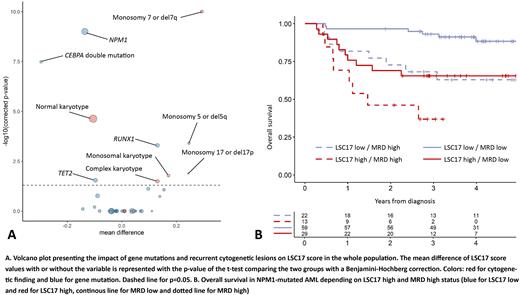
A higher LSC17 score was positively associated with RUNX1 (median difference [md]=0.13, corrected p-value [q]=0.0005) and negatively correlated with NPM1 mutation (md=-0.14, q<0.0001) and double-mutated CEBPA (md=-0.30, q<0.0001). Regarding recurrent cytogenetic lesions, presence of monosomy 7 or del7q (md=0.29, q<0.0001), monosomy 5 or del5q (md=0.25, q=0.0004), monosomy 17, del17p or abnormal 17p (md=0.24, q=0.01), monosomal karyotype (md=0.17, q=0.02) and complex karyotype (md=0.13, q=0.03) were each associated with higher LSC17, whereas normal karyotype was associated with a lower LSC17 score (md=-0.11, q<0.0001) (Figure 1A). In the subgroup of NPM1-mutated AML (n=187), a higher LSC17 score was only correlated with FLT3-ITD (md=0.13, q=0.0002) but not DNMT3A (md=-0.0007, q=1) mutations. Patients were dichotomized into LSC17-low (n=252) and LSC17-high (n=252) groups using the median LSC17 score as cutoff.
Overall, 400 (79%) patients achieved complete response (CR) or CR with incomplete platelet recovery (CRp) after one cycle of induction and an additional 31 (6%) after a second course. LSC17-high patients had a lower rate of CR/CRp after one course of induction (69% vs 89% in LSC17-low patients) in univariate (p<0.0001) or multivariate analysis (OR=0.41, p=0.001) considering ELN17 risk, age and white blood cell (WBC) count. LSC17-high patients had lower 3-year overall survival (OS) from CR/CRp (60.6%, 95% CI, 54.2-67.8%) than LSC17-low patients (75.4%, 95% CI, 70.0-81.2%, p=0.0003). In multivariate analysis considering ELN17, age and WBC, LSC17-high population had shorter DFS (HR=1.39, p=0.038) and a trend for shorter OS from CR/CRp (HR=1.40, p=0.069). In univariate analyses, LSC17-high status was associated with a higher cumulative incidence of relapse (p=0.002) without impact on non-relapse mortality (p=0.27). Considering hematopoietic cell transplantation (allo-HCT) as a time-dependent covariate, there was no interaction on OS from CR/CRp between allo-HCT in first CR and LSC17-high status (p=0.84).
In NPM1-mutated patients (n=187), the rate of CR/CRp after one induction course was 90% in LSC17-high patients versus 96% in LSC17-low patients (p=0.20). In 123 NPM1-mutated patients in CR after the first induction course and with available MRD, LSC17-high status (n=42) predicted a poorer DFS (HR:2.47, p=0.005) in multivariate analysis independently of age (p=0.55), higher WBC (p=0.84), intermediate ELN17 risk (p=0.97) and MRD positivity (p=0.0002). LSC17-high status also independently predicted poorer OS from CR/CRp (HR:2.73, p=0.007) in a similar multivariate model. Three-year OS from CR was 93.1% (95% CI, 86.8-99.9%) in the 59 (48%) NPM1-mutated LSC17-low patients achieving negative MRD compared to 60.7% (95% CI, 49.8-74.0%) in those (n=64, 52%) with LSC17-high status and/or positive MRD (p=0.0001) (Figure 1B).
Conclusion. The LSC17 score complements genetic risk stratification in AML patients treated intensively. Combining LSC17 and MRD assessment identifies a subset of NPM1-mutated AML with excellent long-term prognosis.
Disclosures
Dumas:Janssen: Honoraria; BMS Celgene: Honoraria; Abbvie: Honoraria; Jazz Pharmaceuticals: Honoraria; Daiichi-Sankyo: Honoraria; Astellas: Honoraria. Recher:Takeda: Membership on an entity's Board of Directors or advisory committees; AbbVie, Amgen, Novartis, BMS-Celgene, Jazz Pharmaceuticals, Agios, MaatPharma, Astellas, Roche, Iqvia, Daiichi-Sankyo: Research Funding; AbbVie, Janssen, Jazz Pharmaceuticals, Novartis, BMS-Celgene, Otsuka, Astellas, Daiichi-Sankyo, Macrogenics, Roche, Takeda, Servier, Pfizer: Other: Advisory role; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Lambert:Astellas: Honoraria. Dombret:Incyte: Honoraria; Servier: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal